
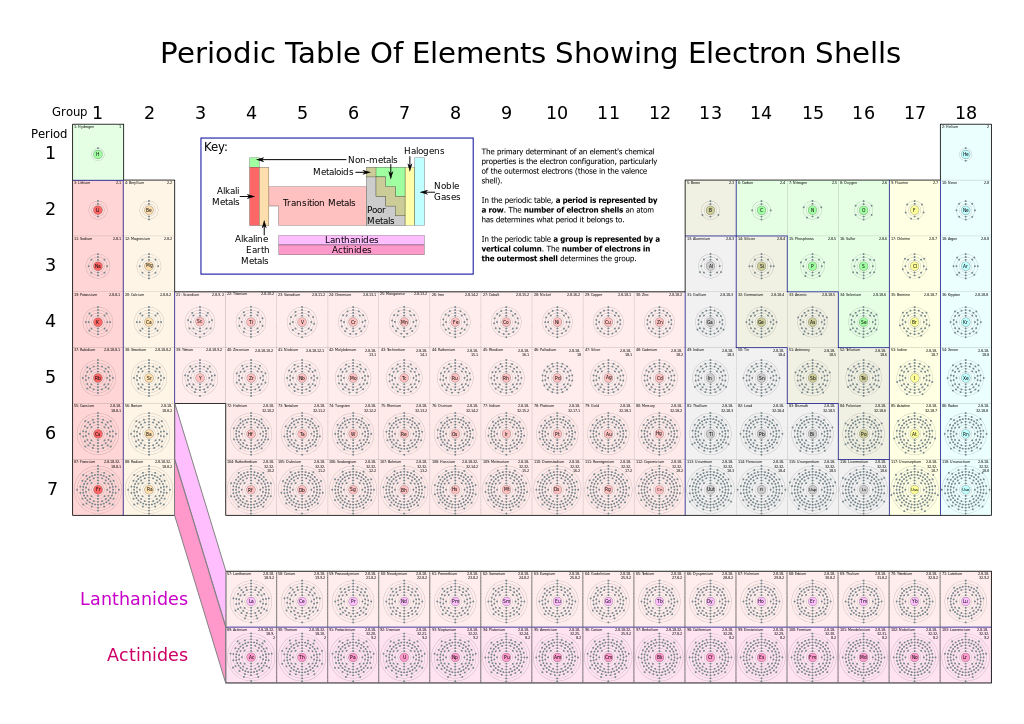
How do you write the electron configuration for Carbon? For example, oxygen has an atomic number of. The basic particle that constitutes a chemical element is the atom, and each chemical element is distinguished by the number of protons in the nuclei of its atoms, known as its atomic number. Humans need 1018 milligrams of iron each day. Haemoglobin carries oxygen from our lungs to the cells, where it is needed for tissue respiration. A lot of this is in haemoglobin, in the blood. It is a shiny, black or dull grey reactive nonmetal, whose name comes from the Latin word carbo, which means charcoal.It is the 17 th most abundant element on earth and is the first element in the carbon group. The average human contains about 4 grams of iron. Carbon (C) is a chemical element of the periodic table, located in the group 14 and the period 2, and is having the atomic number 6. It is a component of rocks as carbonates of calcium (limestone), magnesium. A chemical element is a chemical substance that cannot be broken down into other substances. Iron is an essential element for all forms of life and is non-toxic. Carbon is present as carbon dioxide in the atmosphere and dissolved in all natural waters. It is found in abundance in the sun, stars, comets, and atmospheres of most planets. The electronic configuration of Carbon will be 1s2 2s2 2p2. Carbon is a Group 14 element and is distributed very widely in nature. Atomic Number: 6 Atomic Weight: 12.0107 Melting Point: 3823 K (3550C or 6422F) Boiling Point: 4098 K (3825C or 6917F) Density: 2.2670 grams per cubic. For quick reference, go to the periodic table chart with names listed alphabetical order. This periodic table of the elements with names, atomic number, symbol and mass is color-coded for easier reference by students and researchers. What is the electronic configuration of Carbon 6? Organizing the elements to help further our understanding was first provided by Dmitri Mendeleev. What is the boiling Point of Carbon in Kelvin?īoiling Point of Carbon in Kelvin is 4300 K. Melting Point of Carbon in Kelvin is 3823 K. What is the melting Point of Carbon in Kelvin? What is the boiling Point of Carbon?īoiling Point of Carbon is 4300 K.

Carbon has 6 electrons out of which 4 valence electrons are present in the 2s2 2p2 outer orbitals of atom. How many valence electrons does a Carbon atom have?Ĭarbon has 4 valence electrons. The element Carbon was discovered by Egyptians and Sumerians in year 3750 BCE. What is the color of Carbon?Ĭarbon is of Black color. It is located in group 14 and period 2 in the modern periodic table. Carbon is the 6 element on the periodic table. What is the position of Carbon in the Periodic Table?Ĭarbon is a chemical element with the symbol C and atomic number 6. Carbon is a chemical element with symbol C and atomic number 6. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the preceding period in square brackets. The abbreviated electronic configuration of Carbon is 2s2 2p2. What is the abbreviated electronic configuration of Carbon? Heres a list of all of the chemical elements of the periodic table ordered by increasing atomic number. The electronic configuration of Carbon is 1s2 2s2 2p2. List of chemical elements - Periodic Table. You can effortlessly find every single detail about the elements from this single Interactive Periodic table.What is the electronic configuration of Carbon? Let me tell you how this Interactive Periodic Table will help you in your studies.ġ). Metals reside on the left side of the table, while non-metals.
#C element periodic table free
Free Gift for you: Interactive Periodic Table The periodic table (also known as the periodic table of elements) is organized so scientists can quickly discern the properties of individual elements such as their mass, electron number, electron configuration and their unique chemical properties. In this way, the elements of the same group show similar chemical properties and they also have the same number of valence electrons. They are soft and can be cut easily with a kitchen knife.Īlso all the elements of group 1 have one valence electron.Īll the elements of group 18 are chemically inert (that means they do not easily react with other elements).Īnd all the elements of group 18 have a complete octet (that means they have 8 electrons in their outer shell).
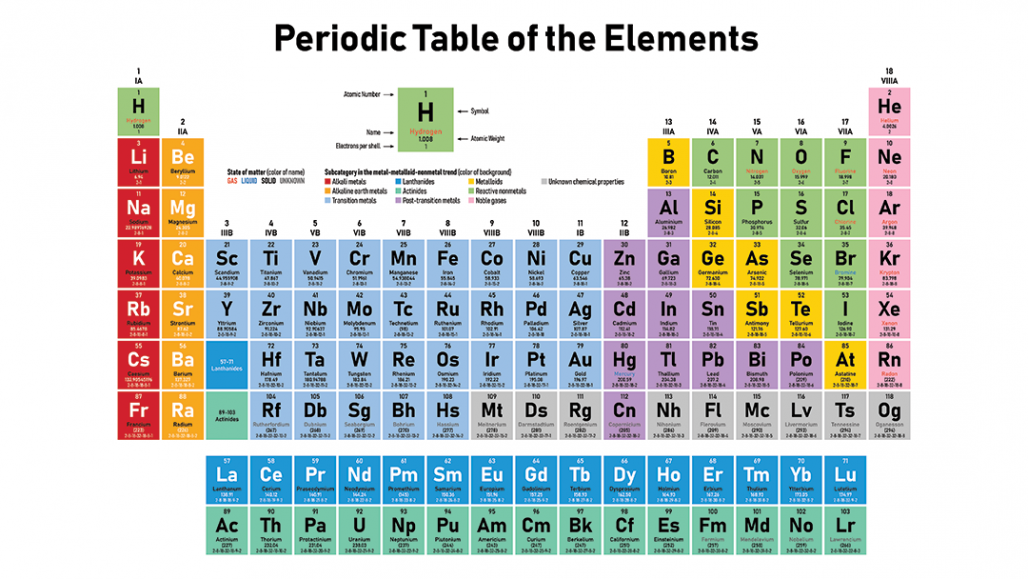
The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.Īll the elements of group 1 are highly reactive to water. There are total 18 vertical columns on periodic table. Groups are the vertical columns on the periodic table.


 0 kommentar(er)
0 kommentar(er)
